We often talk about epilepsy in the plural (epilepsies), because different parameters define the different epileptic syndromes. These parameters include the type of seizure, the cause of the seizure, the symptoms, and the anomalies detected in the electroencephalogram recording (electrical activity produced by the brain).
There can be multiple causes of an epileptic seizure, and there is a distinction between symptomatic epilepsy and idiopathic epilepsy.
Biological mechanisms of epilepsy
An epileptic seizure is abnormally prolonged electrical activity in a set of neurons in the cerebral cortex. The action potential, or nerve impulses, is an electrical message created by an inversion of positive and negative charges on both sides of the neuron membrane. This inversion is caused by an exchange of ions between the cell and its environment (Fig B). At rest, the positive charges are located outside the neuron (Fig A)
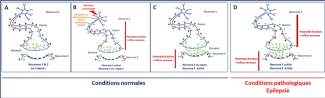
Under normal conditions, each neuron must receive, process and transmit the electrical message to the other neurons via the synapse, through the neurotransmitters (shown as green circles on the diagram).
The neuron that transmitted the electrical signal then begins a repolarization phase. This is where there is a re-inversion of the charges between the outside and inside of the neuron, during which it cannot be activated (Fig C).
If an epileptic seizure occurs, the neurons become hyperexcitable. In other words, a single stimulation leads to a succession, rather than an action potential – a sequence of repetitive action potentials with no rest period (Fig D).
In idiopathic epilepsy, this neuronal hyperexcitability is explained by mutations in the ion channels, located on the neuron membrane, which allow ion exchange and therefore depolarization and repolarization. The membrane generally becomes too permeable, preventing a return to resting potential.
Hyperexcitable neurons make up what is known as the epileptic focus. A distinction is made between focal epileptic seizures, which originate in a very specific region of the brain, and generalized seizures resulting from a sequence of action potentials spread throughout the brain.
During seizures, hyperexcitability is often accompanied by hypersynchrony, where several groups of neurons simultaneously generate action potential at the same rate, amplifying the intensity of symptoms.
At Paris Brain Institute
The “Cellular Excitability and Dynamics of Neural Networks” team, co-led by Stéphane Charpier, Vincent Navarro and Mario Chavez, seeks to understand how the brain becomes epileptic (epileptogenesis), how seizures happen, and to identify the relationships between abnormal electrical activity in a single neuron and the clinical signs observed in patients.
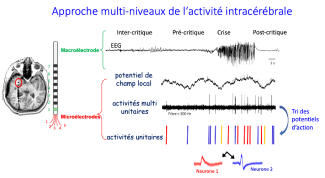
Implanting microelectrodes in the brain during intracerebral EEG exploration of patients with drug-resistant epilepsy is a new approach that makes it possible to monitor brain activity at neuron level during epileptic seizures, but also at a distance (inter-critical period), and shortly before the seizures (pre-critical period).This information is studied at the Epilepsy Unit with the help of Paris Brain Institute’s CENIR-STIM platform, and the data is transferred directly to the Institute’s servers for analysis by Professor Vincent Navarro’s team. This makes it possible to continuously monitor, over a number of days, the activity of groups of neurons: Paris Brain Institute is the only institute in France that has a recording procedure of this kind.
The brain consumes a large part of our daily energy intake. Synapses, which connect neurons to each other, consume a lot of energy in particular. Each time the neurons communicate with each other, a lot of energy is consumed. Unsurprisingly, not having enough energy to maintain communication between neurons has detrimental effects.
The aim of Jaime de Juan-Sanz’s team is to understand and identify the key molecular mechanisms involved in keeping the synapses’ bioenergy at normal levels and to show a relationship between energy dysfunction and an epileptic seizure.