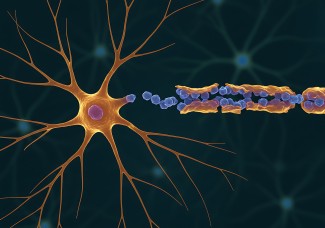
An unprecedentedly precise exploration of the molecular and electrophysiological basis of a form of autoimmune encephalitis, conducted by the "Cellular Excitability and Neuronal Network Dynamics" team at the Paris Brain Institute, elucidates for the first time a scenario for the onset of epileptic seizures in this pathology. The results, published in Progress in Neurobiology, pave the way for the identification of new therapeutic targets for drug-resistant epilepsy.
Epileptic seizure and autoimmune encephalitis
Autoimmune encephalitis occurs as a result of an attack on the central nervous system by an individual's own immune cells. It is an important cause to look for in adult and childhood epilepsy, once the classic seizure triggers have been excluded: an autoimmune cause is thought to be involved in up to 30% of cases. The identification of an autoimmune cause also has major implications for medical treatment. In this case, anti-inflammatory drugs may be effective in treating the symptoms, while conventional anti-epileptic drugs are ineffective. Prompt treatment of autoimmune seizures is crucial because the neuronal hyperactivity associated with seizures can lead to significant cognitive and neurological consequences.
In autoimmune encephalitis, several proteins can be targeted by immune cells, such as NMDA receptors, leading to very severe neuropsychiatric disorders, or the synaptic protein LGI1, the most common target in patients with autoimmune epilepsy, which modulates the activity of the potassium channel KV1.1 at the synapse.
A new study model, combined with unprecedented cellular exploration
How can antibodies targeting the LGI1 protein induce such severe epileptic seizures? To explore this question, Paul Baudin, first author of the study, led by Vincent Navarro (Sorbonne University, AP-HP), associated with Séverine Mahon (Inserm) and Stéphane Charpier (Sorbonne University) in the team "Cellular Excitability and Dynamics of Neuronal Networks", blocked the KV1.1 channel in the motor cortex of rats. This resulted in seizures identical to those observed in patients. By analysing brain activity using electroencephalography (EEG), the researchers were able to identify a specific wave in the motor cortex preceding the onset of the seizure. These initial data reinforce the major homology between the patients and the animal model of encephalitis targeting the LGI1 protein, by blocking the KV1.1 channel.
Using this model, the researchers conducted an in-depth exploration at the cellular level of the consequences of blocking the KV1.1 channel. Combining multi-electrode recordings at the level of the motor cortex, set up with Delphine Roussel from the electrophysiology platform of the Paris Brain Institute, EPhys, and intracellular recordings, the expertise of Stéphane Charpier and Séverine Mahon in the team, they identified major changes in the activity of the synapses and the excitability of the neurons.
A scenario for the onset of seizures
Thanks to this unique approach, the researchers were able to propose a scenario for the onset of seizures and their very frequent repetition. After a first seizure, the neurons are inhibited and then gradually depolarise, i.e. the levels of activation and synchronisation of the neurons are increasingly high, until a new seizure is initiated. The study of the intrinsic characteristics of the neurons shows that they have become hyperexcitable, i.e. much more sensitive to the slightest stimulation. These data also explain the increase in the frequency of seizures in patients over time.
The Paris Brain Institute team suggests a central role for potassium channels in epilepsy associated with antibody-related autoimmune encephalitis. This result, for Prof. Vincent Navarro, opens up major perspectives for therapeutic research, notably on the anti-epileptic potential of potassium channel modulators.
Sources
https://pubmed.ncbi.nlm.nih.gov/35283238/
Baudin P, Whitmarsh S, Cousyn L, Roussel D, Lecas S, Lehongre K, Charpier S, Mahon S, Navarro V. Prog Neurobiol. 2022 Mar 10