Huntington's disease, a rare hereditary neurological disorder, is associated with an energy deficit that precedes the onset of symptoms and is closely linked to their progression. At Paris Brain Institute, Fanny Mochel and her colleagues are testing a synthetic oil, triheptanoin, which can potentially compensate for this deficit. In a phase II trial, the results of which are published in the journal Neurology, the researchers show that this compound had an unexpected effect on the progression of clinical signs.
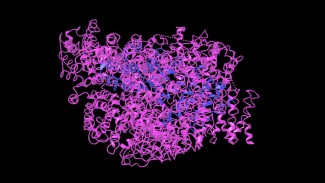
Huntingtin is a protein essential for embryonic development and the formation and maintenance of brain tissue. It is expressed throughout the body. Credit: National Center for Biotechnology Information.
A hereditary neurodegenerative disorder, Huntington's disease is linked to a genetic anomaly in the HTT gene, leading to severely disabling motor, psychiatric, and behavioral disorders that typically appear between the ages of 30 and 50. Currently, available treatments target patients’ symptoms but do not modify the course of the disease. Meanwhile, gene therapy programs are under development.
Several non-neurological symptoms can be detected in the presymptomatic stage, such as significant weight loss associated with lower levels of certain amino acids in the blood. These clinical signs indicate that the body is attempting to compensate for a cerebral energy deficit, likely caused by multiple factors.
Indeed, huntingtin—the protein deficient in the disease—plays a role at various levels in maintaining the body's energy balance. To help the body find the energy necessary for proper brain function without depleting its reserves, Fanny Mochel and her team proposed using a drug with promising properties.
“Triheptanoin is a medium-chain triglyceride (MCT) developed about twenty years ago. It’s a type of fatty acid that can enter cells directly. Unlike dietary fatty acids, it produces two different types of energy molecules. Its energy yield is, therefore, particularly high, especially for brain cells”, the researcher continues.
A Disease-Modifying Treatment?
After several highly encouraging studies in animals and humans, Fanny Mochel and her team recruited 107 French and Dutch patients in the early stages of the disease in collaboration with Raymund Roos’s team in the Netherlands. Initially conducted as a six-month randomized, double-blind clinical trial, the study was extended by an open-label phase lasting six months, during which patients were compared to the placebo arm of a similar therapeutic trial.
Participants were evaluated at 6 and 12 months using the Unified Huntington’s Disease Rating Scale (UHDRS), a standardized tool that tracks the progression of motor, cognitive, and behavioral abilities in patients. They also underwent imaging exams to assess atrophy of the caudate nucleus in the striatum—a brain region highly sensitive to biological changes associated with the disease.
The 12-month study results showed that the symptoms of patients who received triheptanoin had barely progressed, unlike those who received the placebo, whose condition deteriorated as expected. Moreover, the progression of caudate nucleus atrophy was reduced by 50% in patients who received triheptanoin, indicating a direct effect of the treatment on the disease mechanisms.
Promising Results Await Confirmation
“We observed a significant clinical benefit from triheptanoin, which has the advantage of being an accessible, minimally invasive treatment with good patient acceptance, as it is administered as an oil mixed with food,” says Fanny Mochel.
The researchers are now awaiting a phase III clinical trial to determine whether triheptanoin improves motor, cognitive, and behavioral symptoms after prolonged use for a year or more. According to them, this treatment cannot cure the disease entirely, but it may be one of the compounds that can help slow its progression and improve patients' daily lives.
Funding
This study was funded par Ultragenyx Pharmaceutical Inc.
Sources
Mochel, F., et al. Phase 2 randomized trial of Triheptanoin for patients in early-stage Huntington disease. Neurology, Décembre 2024. DOI : 10.1212/WNL.0000000000210194.
The team aims to elucidate the role of innate and adaptive immune cells in myelin destruction and repair in multiple sclerosis, target innate immunity as therapeutic mediators in neurometabolic diseases, and decipher the code to control macrophage...
Read more