Brain plasticity is the dynamic process happening in our brain as we learn through experience, and it varies greatly with age. What are the mechanism behind this? A study conducted by Alberto Bacci’s team at the Institut du Cerveau - ICM discovered the mechanism behind cortical sensory plasticity. These results were published in eLife.
Brain plasticity is the dynamic process happening in our brain as we learn through experience, and it varies greatly with age. What are the mechanism behind this? A study conducted by Alberto Bacci’s team at the Institut du Cerveau - ICM discovered the mechanism behind cortical sensory plasticity. These results were published in eLife.
In the cerebral cortex, sensory-motor plasticity is at the base of our capacity to learn things and skills. This process is accompanied by circuit rewiring in the brain, and is particularly present in specific developmental windows, known as the “critical periods”, during which neuronal circuits adapt to the flow of sensory input received from the external world. These time windows for sensory-motor plasticity are crucial, for example, to learn perfectly a new language, or to achieve excellence in violin or football.
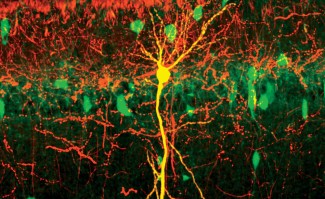
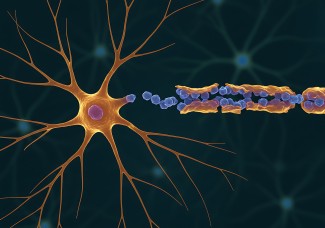
Critical periods in the cerebral cortex are transitory periods, which are closed by several molecular players. Among those, extracellular perineuronal nets (PNN) play a major role in closing brain plasticity. PNNs are part of the extracellular matrix, and are composed of proteins and complex sugars linked to one another. PNNs form a dense net around only a specific type of inhibitory neuron, the parvalbumin (PV) basket cells. These nets accumulate around PV cells only at the end of a critical period, and, by doing so, prevent plasticity in adult subjects. Importantly, PV basket cells synchronize the activity of groups of neurons, creating rhythmic patterns of neural impulses. In the visual cortex, these patterns are the brain's way of representing incoming information from the eyes.
Dissolving PNNs with an enzyme leads to a reopening of cortical plasticity in adults, but the exact mechanism remains to be elucidated.
Faini et al. investigated this mechanism in the context of visual cortical plasticity. This type of plasticity can modulate the ocular dominance bias, the fact that the right hemisphere of the brain receive more sensory inputs from the left eye and vice versa. Closing one eye during the critical period, leads to the loss of this contralateral bias, due to plastic changes induced by reduced sensory inputs from the closed eye. Adults cannot change ocular dominance, unless PNNs are removed. But what are the mechanisms?
We found thatPNNs selectively “muffle” inputs onto PV cells from the thalamus, which is a major relay of sensory information in the brain. PNN accumulation around PV cells reduces the strength of incoming signals from the eyes when they reach these neurons in the visual cortex. Disrupting the nets enhances visual signals onto PV cells only, thus enabling cortical circuits of adult mice to behave as they did during the postnatal critical period.
All these effects were strongly reduced by sensory deprivation, namely preventing seeing out of one eye, indicating that the effect of PNNs on thalamic input onto PV cells depends primarily on visual sensory experience.
Overall, these results unravel the mechanism underlying PNN-dependent re-opening of cortical plasticity. After the critical period, PV cells grow PNNs as a protection to prevent too strong inputs coming from the thalamus. But this protection is achieved at the cost of plasticity. This mechanism is consistent with the fact that plasticity, while essential during development for integrating all kind of experiences and skills, may have to be partially given up to consolidate our internal model of what we see, hear or experience.
It could also have a major importance for pathologies characterized by altered sensory perception such as schizophrenia and autism. Indeed, independent studies revealed that PNN accumulation around PV cells is altered in these brain disorders. Investigating the roles of these nets in more detail could therefore help researchers to develop new treatments for such conditions. More widely, understanding precisely how cortical circuits lose their ability to rewire themselves improves our knowledge of how we learn and store memories.
This work was done in collaboration with a group in Pisa, Italy.
Sources
https://pubmed.ncbi.nlm.nih.gov/30561327/
Faini G, Aguirre A, Landi S, Lamers D, Pizzorusso T, Ratto GM, Deleuze C, Bacci A. Elife. 2018 Dec 18