Products derived from the intestinal microbiota enter the bloodstream and modulate the host's physiological processes, such as immunity, metabolism, and brain function. Scientists from the Institut Pasteur (a research partner of Université Paris Cité), the Paris Brain Institute, Inserm and CNRS have discovered in an animal model that neurons in the hypothalamus directly detect variations in bacterial activity and adapt appetite and body temperature accordingly. These results show the existence of a direct dialogue between the gut microbiota and the brain, a discovery that could be exploited for new therapeutic approaches against metabolic disorders such as diabetes or obesity. These results will be published in Science on 15 April 2022.
The gut microbiota is the largest reservoir of bacteria in the body. A growing body of research shows how dependent the host and its gut microbiota are on each other and highlights the importance of the gut-brain axis.
At the Institut Pasteur, neurobiologists from the Perception and Memory unit, immunobiologists from the Microenvironment and Immunity unit, and microbiologists from the Biology and Genetics of the Bacterial Wall unit, in association with the “Structural Dynamics of Networks” of the Paris Brain Institute, have pooled their expertise to understand how gut bacteria can have a direct effect on the activity of certain brain neurons.
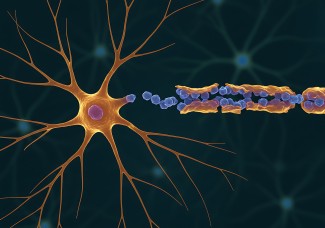
The scientists were particularly interested in the NOD2 (Nucleotide Oligomerization Domain) receptor, which is present inside cells, in particular immune cells. This receptor detects the presence of muropeptides, compounds of the bacterial walls, which can be considered as by-products of the intestinal microbiota.
Furthermore, it was already known that variants of the gene coding for the NOD2 receptor are associated with certain diseases of the digestive system, such as Crohn's disease, but also with certain neurological diseases or mood disorders.
These data did not yet allow to conclude that there is a direct relationship between the functioning of brain neurons and bacterial activity in the gut. This is what the consortium of scientists has brought to light in this new study.
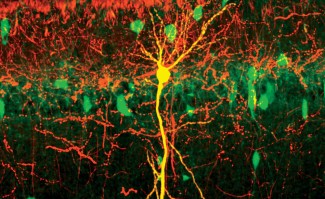
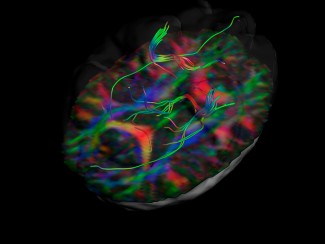
Using brain imaging techniques, the scientists first observed in mice that the NOD2 receptor is expressed by neurons in different regions of the brain, and in particular in a centre called the hypothalamus. They then discovered that these same neurons have their electrical activity repressed when they encounter bacterial muropeptides from the intestine. Muropeptides are released by bacteria as they proliferate. "Muropeptides in the gut, blood and brain are considered to be markers of bacterial proliferation," explains Ivo G. Boneca, head of the Biology and Genetics of the Bacterial Wall unit at the Pasteur Institute (CNRS/Inserm).
Conversely, in the case where the NOD2 receptor is defective, these neurons are no longer repressed by muropeptides; the brain then loses control over food intake and body temperature.
As a result, the mice gain weight and are more likely to develop type 2 diabetes, especially in older females.
Surprisingly, the scientists have shown that it is the neurons that directly perceive the bacterial muropeptides, whereas this task is usually assigned to the cells of the immune system. "It is amazing to discover that bacterial fragments act directly on a nerve centre as strategic as the hypothalamus, which is known to manage vital functions such as body temperature, reproduction, hunger and thirst," comments Pierre-Marie Lledo, CNRS researcher and head of the Perception and Memory unit at the Institut Pasteur.
Thus, the neurons seem to detect bacterial activity (proliferation and death) to directly measure the impact of food intake on the intestinal ecosystem. "It is possible that an excessive food intake or a particular food may encourage the exaggerated expansion of certain bacteria or pathogens, thus endangering the intestinal balance," emphasizes Gérard Eberl, head of the Microenvironment and Immunity Unit at the Institut Pasteur (Inserm).
Given the impact of muropeptides on hypothalamic neurons and metabolism, it is possible to question their role in other brain functions, and thus understand the association between certain brain diseases and NOD2 genetic variants. This discovery opens the way to new interdisciplinary projects for the research teams and, in the long term, to new therapeutic approaches against brain diseases or metabolic diseases such as diabetes and obesity.
Sources
https://pubmed.ncbi.nlm.nih.gov/35420957/
Gabanyi I, Lepousez G, Wheeler R, Vieites-Prado A, Nissant A, Wagner S, Moigneu C, Dulauroy S, Hicham S, Polomack B, Verny F, Rosenstiel P, Renier N, Boneca IG, Eberl G, Lledo PM. Science. 2022 Apr 15