Certain patients with multiple sclerosis (MS) can partially regenerate myelin—the protective sheath that surrounds nerve fibers—which is damaged during the evolution of the disease. In studying how immune cells influence this remyelination, researchers from Paris Brain Institute, led by Violetta Zujovic, have made a key discovery: a metabolic dysfunction in the patients’ macrophages. These immune cells are supposed to clear myelin debris and facilitate its repair. What if this issue could hinder regeneration? These findings, published in Neurology, Neuroimmunology & Neuroinflammation, pave the way for a better understanding of patients’ recovery potential.
Multiple sclerosis is an inflammatory disease in which immune cells invade the central nervous system and destroy myelin, a vital component for nerve signal transmission. However, under certain conditions, a specific type of these cells—macrophages—can reverse the damage by contributing to remyelination.
“In clinical trials, we observe that some patients exhibit significant remyelination capacity, while others do not. We wondered if these tendencies were linked to the characteristics of macrophages and their response to signals in their environment”, Violetta Zujovic (Inserm), head of the “Myelin Plasticity and Regeneration” team at Paris Brain Institute, and coordinator of the study, explains.
To investigate, the researchers recruited 47 MS patients and 46 healthy controls. They collected monocytes, a type of immature white blood cell, from participants. “These naive cells, freshly produced in the bone marrow, are unaffected by the disease environment—allowing us to study their behaviour under strictly controlled conditions”, the researcher adds.
Inflammation or Regeneration?
The monocytes were exposed to signals mimicking the brain environment to differentiate them into macrophages, enabling researchers to observe how these immune cells regulate pro-inflammatory and pro-regenerative genes.
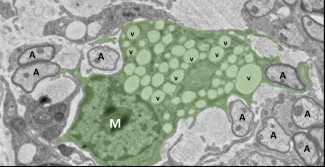
The study revealed significant differences in the behaviour of monocytes from MS patients compared to controls: a heightened inflammatory response; a reduced capacity to clear myelin debris, a critical step for initiating repair; and a failure to effectively induce precursor cells of oligodendrocytes—which are responsible for generating new myelin sheath—to differentiate.“Regardless of the signals we provided to the immune
cells from patients—including those aimed at activating regeneration—they expressed pro-inflammatory proteins”, Zujovic notes. “Now, we know why: patients’ macrophages have a metabolic anomaly. They struggle to properly use their mitochondria, the microscopic powerhouses that provide energy to the cell.”
Immune History’s Role in MS
This discovery sheds light on how exaggerated inflammatory responses and metabolic dysfunctions in macrophages contribute to the pathological mechanisms of multiple sclerosis. Indeed, these issues may stem from specific events in a patient’s immune history, such as viral exposure.
This hypothesis aligns with current theories suggesting that MS results from a combination of an infection—particularly with the Epstein-Barr virus—and a genetic predisposition. Immune cells, marked by past encounters with one or more viruses, may respond inappropriately to new signals, leading to chronic inflammation.
The influence of immune memory has already been demonstrated in autoimmune diseases like lupus or rheumatoid arthritis. This is the first time it’s been highlighted in a neurological disease.
New Avenues for Research
Future studies aim to identify the exact combination of events that precede the onset of MS. Advanced imaging tools, such as PET-MRI, could help analyze patients’ profiles individually to determine whether immune cell dysfunction correlates with the progression of myelin lesions and disability over time.
Ultimately, this investigative method could be extended to other neurological disorders, such as Alzheimer’s disease, where amyloid plaques accumulate in the brain. Macrophages also play a role in clearing these plaques—though with varying degrees of efficiency—raising another puzzle to be solved.
Funding
This study was funded by the OCIRP Foundation, the Bouvet-Labruyère Prize, the Sanofi Innovation Awards program, and the Investments for the Future program of the French National Research Agency.
Sources
Fransson, J., et al. Multiple sclerosis patient macrophages impaired metabolism leads to an altered response to activation stimuli. Neurology, Neuroimmunology & Neuroinflammation, Octobre 2024.
DOI : 10.1212/NXI.0000000000200312.
The team aims to elucidate the role of innate and adaptive immune cells in myelin destruction and repair in multiple sclerosis, target innate immunity as therapeutic mediators in neurometabolic diseases, and decipher the code to control macrophage...
Read more
Multiple Sclerosis (MS)
Multiple sclerosis (MS) is the second leading cause of acquired disability in young adults, after injury. It affects 120,000 people in France today, with 3,000 new cases diagnosed each year. This makes it a major public health issue, since it also...
Read more