A recent study carried out by Frédéric Darios in the team led by Alexis Brice at the Institut du Cerveau - ICM highlights the importance of spatacsin, a protein affected in pathologies such as hereditary spastic paraplegia.
Hereditary spastic paraplegia, or HSP, is a diverse group of diseases in terms of clinical symptoms as well as on a genetic and neuropathological level. HSPs are the second most frequent group of illnesses affecting motoneurons, responsible for transferring nerve signals to muscles.
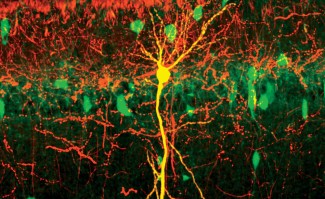
From a clinical perspective, these illnesses are characterized by progressive weakening of the lower limbs along with spasticity, a type of muscle stiffness, and loss of sensitivity. These symptoms are primarily due to degeneration of the corticospinal tract (neurons are located in the motor cortex and their axons project to the spinal cord), which controls voluntary movement.
The primary cause of hereditary spastic paraplegia is mutation of gene SPG11. These types of mutations are also linked to rare forms of amyotrophic lateral sclerosis (ALS) and Charcot-Marie-Tooth (CMT) disease.
SPG11 codes for spatacsin, a protein involved in various cellular mechanisms. The vast majority of mutations identified in patients appear to lead to loss of function of spatacsin. What happens in this case? This is what Institut du Cerveau - ICM researchers set out to discover.
To do so, they used a mouse model that replicates the most frequent SPG11 mutation. Mice with non-functioning SPG11 genes display the same group of symptoms as patients with SPG11 mutation-induced HSP, ALS and CMT:
- Early onset of motor and cognitive disorders.
- Progressive cerebral atrophy with neuron loss in several areas of the brain and spinal cord as well as an accumulation of defective axons in the corticospinal tract.
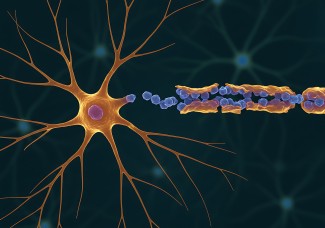
- Neuromuscular junction alterations and muscular atrophy.
Based on this model, researchers focused on the effects of loss of spatacsin at the cellular level. Results show that this loss alters function of lysosomes, degeneration “factories” inside cells. Consequently, cells can no longer get rid of degradation by-products and lipids build up inside lysosomes.
The present study highlights the relationship between neuron degeneration, lysosome malfunction, and lipid metabolism. Many proteins that are coded by affected genes in hereditary spastic paraplegia are also involved in lysosome function and lipid metabolism in a recurring manner. Understanding this mechanism may therefore create new opportunities for future treatment.
Sources
Loss of spatacsin function alters lysosomal lipid clearance leading to upper and lower motor neuron degeneration. Branchu J, Boutry M, Sourd L, Depp M, Leone C, Corriger A, Vallucci M, Esteves T, Matusiak R, Dumont M, Muriel MP, Santorelli FM, Brice A, El Hachimi KH, Stevanin G, Darios F.
http://www.sciencedirect.com/science/article/pii/S0969996117300359