The anomalies underlying neurodegenerative diseases may arise during development—decades before the first symptoms appear. This hypothesis is gaining traction thanks to a new study published in Nature Communications. According to researchers from the Paris Brain Institute, the quality of mitochondria—the cell’s energy powerhouses—is tightly regulated by a specific genetic program that shapes neuronal circuits and, in the long term, determines brain health.
The normal functioning of the brain depends on a complex network of neuronal populations organized into circuits. This delicate architecture forms during development under the influence of carefully coordinated genetic and cellular programs.
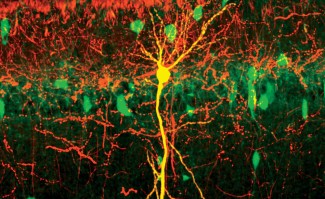
Among the key players in these processes, mitochondria play a central role—especially those located in axons, the long nerve fibers that connect neurons to one another. Mitochondria are responsible for producing ATP, the energy required for cellular activity. But they also regulate local calcium concentrations, support synapse formation—the communication points between neurons—and contribute to brain plasticity.
Until now, it was unclear which genetic factors ensured that mitochondria perform their “architectural” work correctly during development. effectuent correctement leur travail d’architecte au cours du développement.
Properly functioning mitochondria for harmonious development
To learn more, Iryna Mohylyak, a postdoctoral researcher, and her colleagues from Paris Brain Institute and the University of Louvain studied the visual system of the fruit fly, a model particularly suited to investigating the molecular mechanisms that precede brain circuit formation.
“We discovered a genetic regulatory program that controls mitochondrial quality during a specific period of neurodevelopment. When this program is switched off, axonal mitochondria become abnormally large, and neuronal connections deteriorate significantly,” explains Mohylyak.
This program relies on a transcription factor—named Mirana (for mitochondrial integrity regulator of neuronal architecture)—a protein that regulates the expression of several genes. Mirana is transiently activated during circuit formation, controls mitochondrial shape, and prepares neurons to establish stable, efficient connections throughout life.
Mirana is specific to the fruit fly, but the team identified its mammalian counterpart: the transcription factor TZAP (Telomere-Associated Protein). In mice, loss of TZAP drastically reduces neurotransmitter release in the hippocampus.
We already knew TZAP’s role in cancer biology, but this is the first time it’s been shown to play a part in neural circuit development. The human genetic program is truly fascinating—the same genes can act in very different contexts throughout life.
A link with neurodegenerative diseases
Among the genes regulated by Mirana are PINK1 and PARK2, mutations of which are implicated in early-onset familial forms of Parkinson’s disease.
“The fact that PINK1 is essential for normal brain development while also playing a role in Parkinson’s disease supports a central hypothesis for our team: neurodegeneration doesn’t suddenly appear during aging—it has its roots in neurodevelopment,” explains Hassan.
In fruit flies, temporary inactivation of Mirana and PINK1 is sufficient to permanently alter brain connectivity. Moreover, this defect is not compensated for in adults even if Mirana is reactivated, proving that a brief disruption during this critical window of neurodevelopment can leave a lasting imprint on the brain.
By revealing this genetic program of mitochondrial control, the researchers open a promising avenue for better understanding, and perhaps preventing, brain diseases in which communication between neurons accumulates abnormalities from the embryonic stage.
Sources
Mohylyak, I. et al. Temporal transcriptional regulation of mitochondrial morphology primes activity-dependent circuit connectivity. Nature Communications, Septembre 2025. DOI: 10.1038/s41467-025-62908-2.
Funding
This research was funded by the Investissements d’Avenir (PIA) program, Paris Brain Institute, the French National Research Agency (ANR), the Allen Distinguished Investigator Award, the Roger De Spoelberch Foundation Prize, and the National Institutes of Health (NIH).
Illustration
Reconstruction of part of the nervous system of a fruit fly larva (Drosophila melanogaster). Lateral view of neurons and synapses in the neuropil. Credit: Albert Cardona / Wellcome Images.
The "Brain Development" team, led by Bassem HASSAN, is interested in the formation of neurons and neural networks during brain development thanks to models of Drosophila and murine flies. The team is studying the transcriptional control of embryonic...
Read more