There is currently no cure for Alzheimer's disease. The treatments available in France—which are not reimbursed—are known as symptomatic treatments, meaning that they act on the consequences of the disease rather than its underlying cause. In 2018, following recommendations from the HAS and due to insufficient data on their long-term benefits and safety, the Ministry of Health decided to stop reimbursing these anti-Alzheimer's drugs.
Teams from the Lille Memory Resource and Research Center, the Lille Neuroscience & Cognition Research Center, and the Department of Public Health Epidemiology, Health Economics and Prevention at Lille University Hospital (CHU de Lille) seized this pivotal moment to compare the progression of cognitive decline across the country in patients who had either stopped or continued their treatment. In collaboration with researchers from the Paris Brain Institute, they demonstrated that acetylcholinesterase inhibitor treatments were associated with moderate but sustained clinical benefit, without increased mortality. These findings were published in The Lancet Regional Health – Europe.
A Moderate but Sustained Cognitive Benefit over the Long Term
During the 2011 reassessment of drugs indicated for the symptomatic treatment of Alzheimer’s disease, experts from the Transparency Commission of the French National Authority for Health expressed concern about the relative lack of long-term efficacy and safety data for acetylcholinesterase inhibitors in real-life patients, who are generally older and have more comorbidities than participants in clinical trials. The absence of convincing answers to these concerns prompted an unfavorable report from the Transparency Commission in 2016, announcing the delisting of these drugs from reimbursement in August 2018—20 years after the market introduction of donepezil, the leading drug in this class.
Simon Lecerf from Lille University Hospital and Octave Guinebretiere from the Paris Brain Institute, supervised by Thomas Nedelec, Stanley Durrleman from the Aramis team at the Paris Brain Institute, and Prof. Thibaud Lebouvier from Lille University Hospital, studied the cognitive progression of 5,700 patients with Alzheimer’s disease who either stopped or continued treatment in 2018. Based on the MMSE (Mini Mental State Examination), a 30-point global cognitive assessment score commonly used to monitor the progression of Alzheimer's disease, the study demonstrates a moderate but sustained cognitive benefit over the long term (four years) among patients who continued treatment (“continuers”), with no difference in mortality compared to those who discontinued (“discontinuers”).
Cognitive progression shows the typical effect of acetylcholinesterase inhibitors on symptoms.
In terms of cognitive performance, the gap between those who continue treatment and those who stop widens very quickly, then the curves remain broadly parallel.
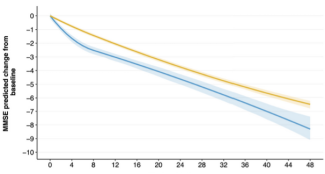
More specifically, the average MMSE difference between discontinuers and continuers was 0.97 points (95% CI 0.68–1.27; p < 0.001) at one year and 1.81 points (0.91–2.71; p < 0.001) at four years. This corresponds to an estimated delay in disease progression of 6.5 months [5.4–7.5] at one year and 11.3 months [7.2–14.5] at four years.
The First Emulated Trial Applied to Alzheimer’s Disease
One of the original aspects of this work lies in its methodology, with the implementation of an emulated trial. This novel approach allows for comparative evaluation of treatment effectiveness in situations where a traditional clinical trial may not be feasible. For this study, researchers used real-life data from the National Alzheimer Database (Banque Nationale Alzheimer)—a unique national database supported by more than 600 memory clinics and backed by the Directorate-General for Healthcare Provision and the Federation of Memory Centers—as well as from the Méotis memory consultation network, supported by the Hauts-de-France Regional Health Agency.
“The idea is to harness the natural variability of observational data to reproduce the conditions of a randomized clinical trial, without the logistical constraints,” explains Thomas Nedelec, researcher at the Paris Brain Institute.
As an observational study, and despite careful adjustment using inverse probability weighting and multiple sensitivity analyses, residual bias cannot be entirely ruled out. Nevertheless, the study confirms the results of the DOMINO trial, a randomized study conducted in the United Kingdom to determine whether anti-Alzheimer drugs could still have a role in the advanced stages of the disease. Published in the New England Journal of Medicine in 2012, this double-blind, placebo-controlled study compared the effects of discontinuing versus continuing treatment on cognitive function and autonomy. Its positive results did not influence the decision to withdraw reimbursement, as the clinical relevance of the findings in very advanced disease stages was considered questionable, and the patients included were not representative of real-life conditions.
“Thanks to this emulated trial, our analysis covers a much larger population than DOMINO, which is more representative of everyday clinical practice, including patients in the early stages of the disease and followed up over a longer period. These findings help address the questions raised by the Transparency Commission, showing sustained long-term effectiveness and apparent safety of acetylcholinesterase inhibitors used in real-world conditions,” says Prof. Thibaud Lebouvier, neurologist and researcher at Lille University Hospital.
Sources
Lecerf, S., et al. Long-term effect of discontinuing anticholinesterase treatment on cognitive decline and mortality in Alzheimer's disease in France: a quasi-experiment and target trial emulation study. The Lancet Regional Health, February 2026. DOI: 10.1016/j.lanepe.2026.101607.
Image
Hippocampal neurons. Credit: Tristan Geiller, Losonczy Lab.
Alzheimer's disease
With longer life expectancies and the biological mechanisms at the origin of neurodegenerative diseases becoming increasingly complex, it is now estimated that, in France in 2020, 1.3 million people were affected by Alzheimer’s disease. Today, more...
Read more
The ARAMIS team, led by Ninon BURGOS & Olivier COLLIOT, aims to build numerical models of brain diseases, particularly neurodegenerative pathologies, from multimodal patient databases. The main approaches used are machine learning (artificial...
Read more